Unify ERP and GMP operations into
one AI Platform
Cut batch release times by 30%, enable 5x faster documentation cycle times, and eliminate manual errors with our AI-powered GMP platform, built by founders who have walked your factory floors and faced your auditors.
One AI platform for global compliance
Testimonials
What Industry Leaders are Saying
Unify ERP, quality and compliance into one AI platform
Purpose built for Life sciences
Why teams switch from spreadsheets and legacy software to Zenopsys
Legacy / Paper
Outdated methods
- Doesn't scale
- High cost for customisations
- Zero visibility
- Lack of Integrations
- High cost of operations
- lengthy manual processes
- AI cannot be implemented
Enterprise Software
Overcomplicated
- Heavy customisation required
- Long Implementation timeline
- Siloed Systems
- Expensive Integrations
- High cost per Seat
- Rigid processes
- Data isn't AI-ready
Zenopsys
Optimized solution
Ready-to-use, user-friendly modules
Go live in 15 days
Unified Platform
In built Integrations
Flat pricing
Flexible processes with AI automation
AI native platform
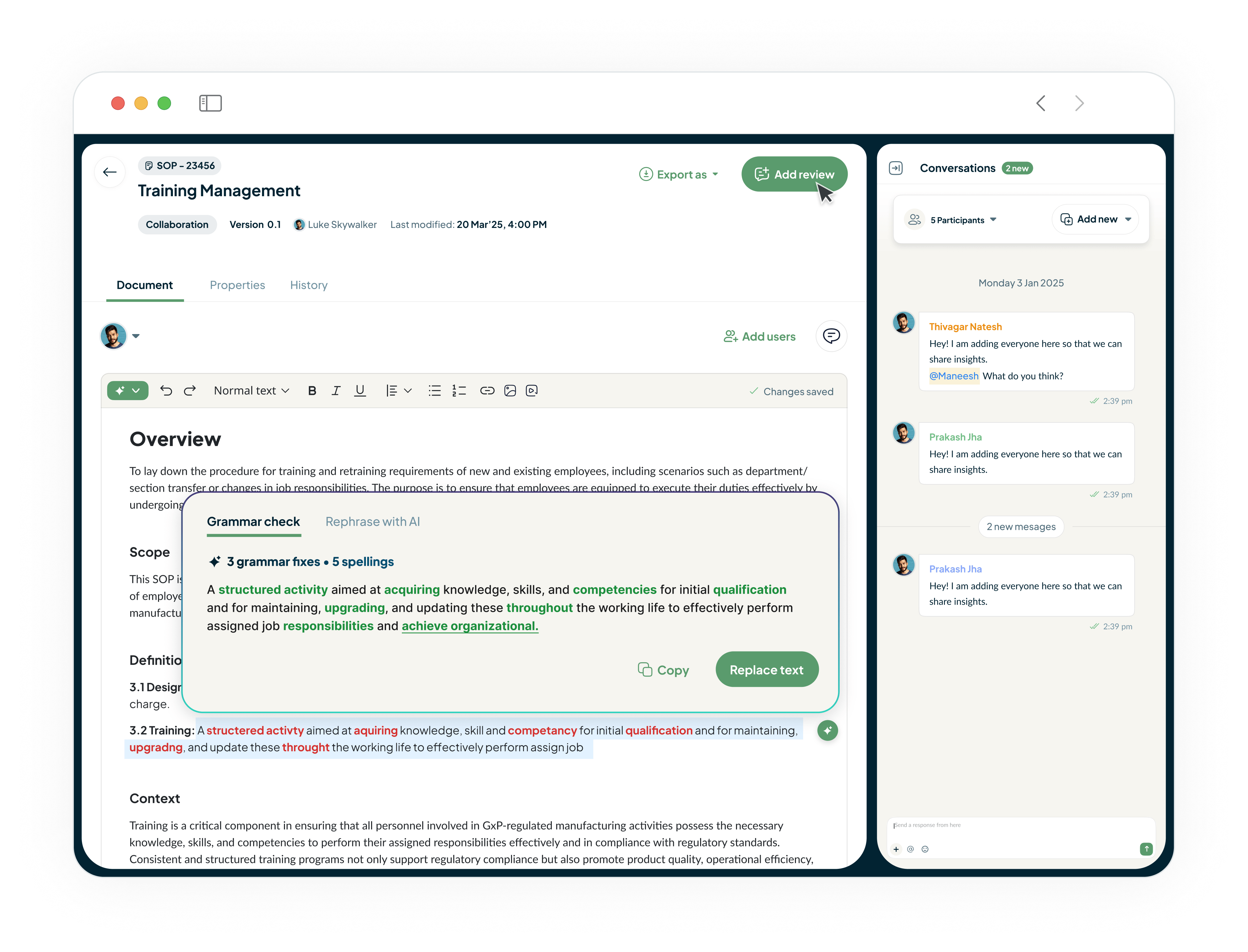
Draft Compliant GxP Documents in Minutes, Not Weeks
Our DMS, featuring an industry-first AI document editor, significantly reduces administrative burden. Generate compliant first drafts of SOPs, Batch Records, or validation protocols with simple prompts, drastically cutting drafting time and ensuring regulatory alignment.
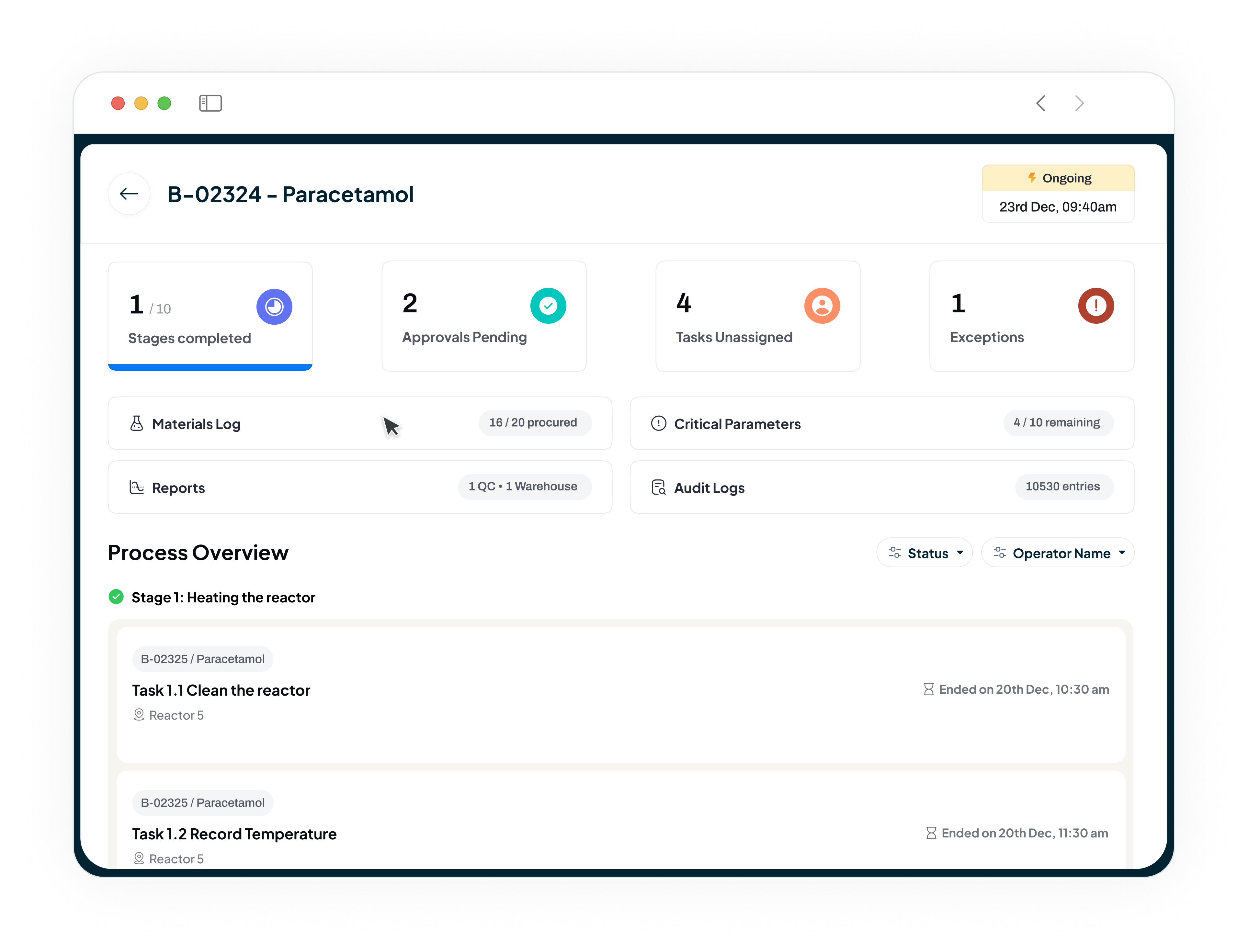
Accelerate Batch Release from Weeks to Days
Our MES replaces paper batch records with electronic batch records (eBRs), enabling "review by exception." This allows your Quality team to focus only on deviations, leading to faster compliant batch releases and improved production cycle times.

Prevent Deviations Before They Happen
Stop relying on manual checks and double-signing. Our MES and Asset Management modules create intelligent process interlocks. This system automatically verifies that operators are following the correct SOP steps, that the right materials are being used, and that only calibrated, validated, and clean equipment can be selected for a batch.
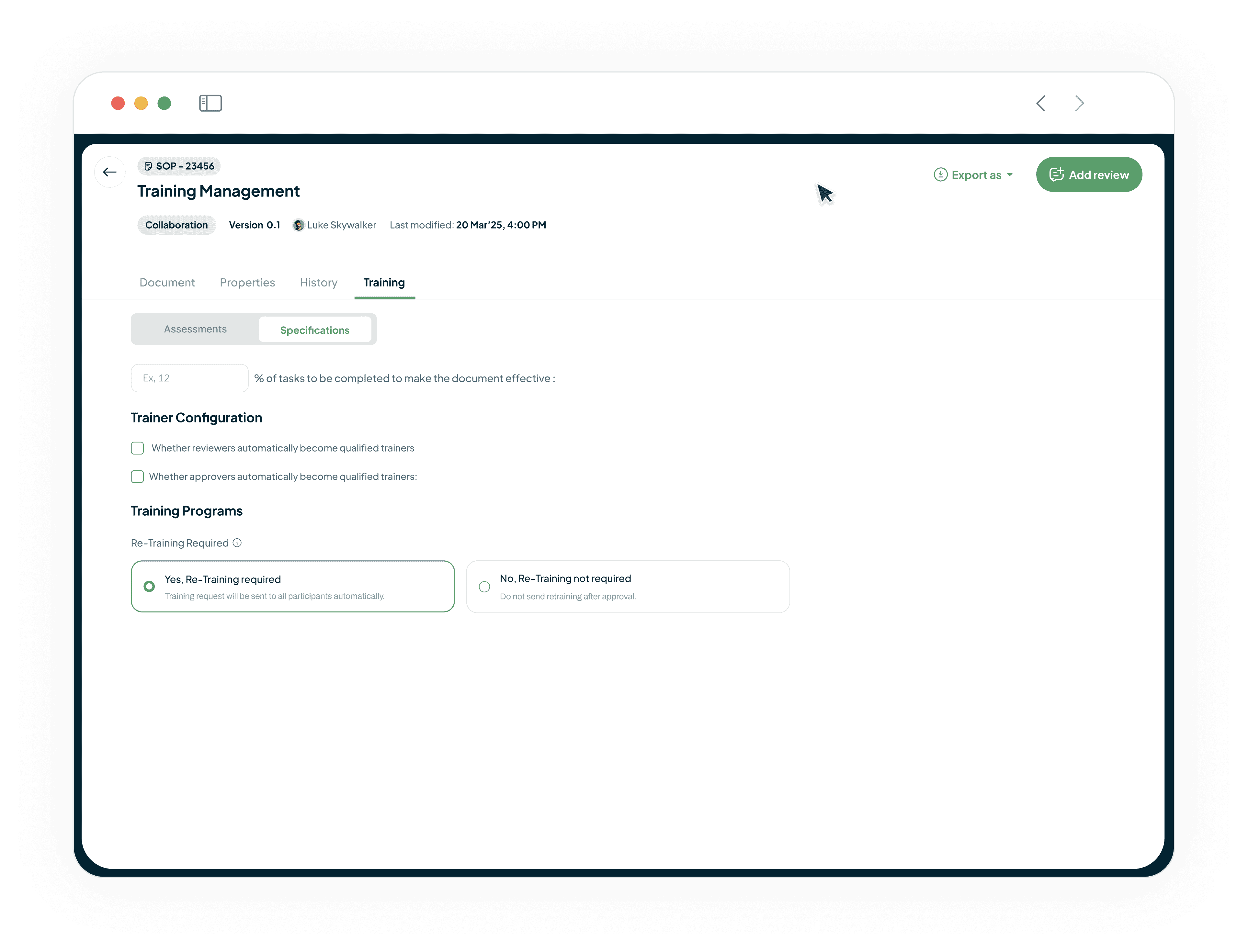
Automatically Close Training Gaps
Our platform links your DMS and LMS. When a new SOP is approved in the DMS, the LMS automatically assigns training to affected roles. This ensures operators are always trained on the latest SOP, guaranteeing continuous compliance.
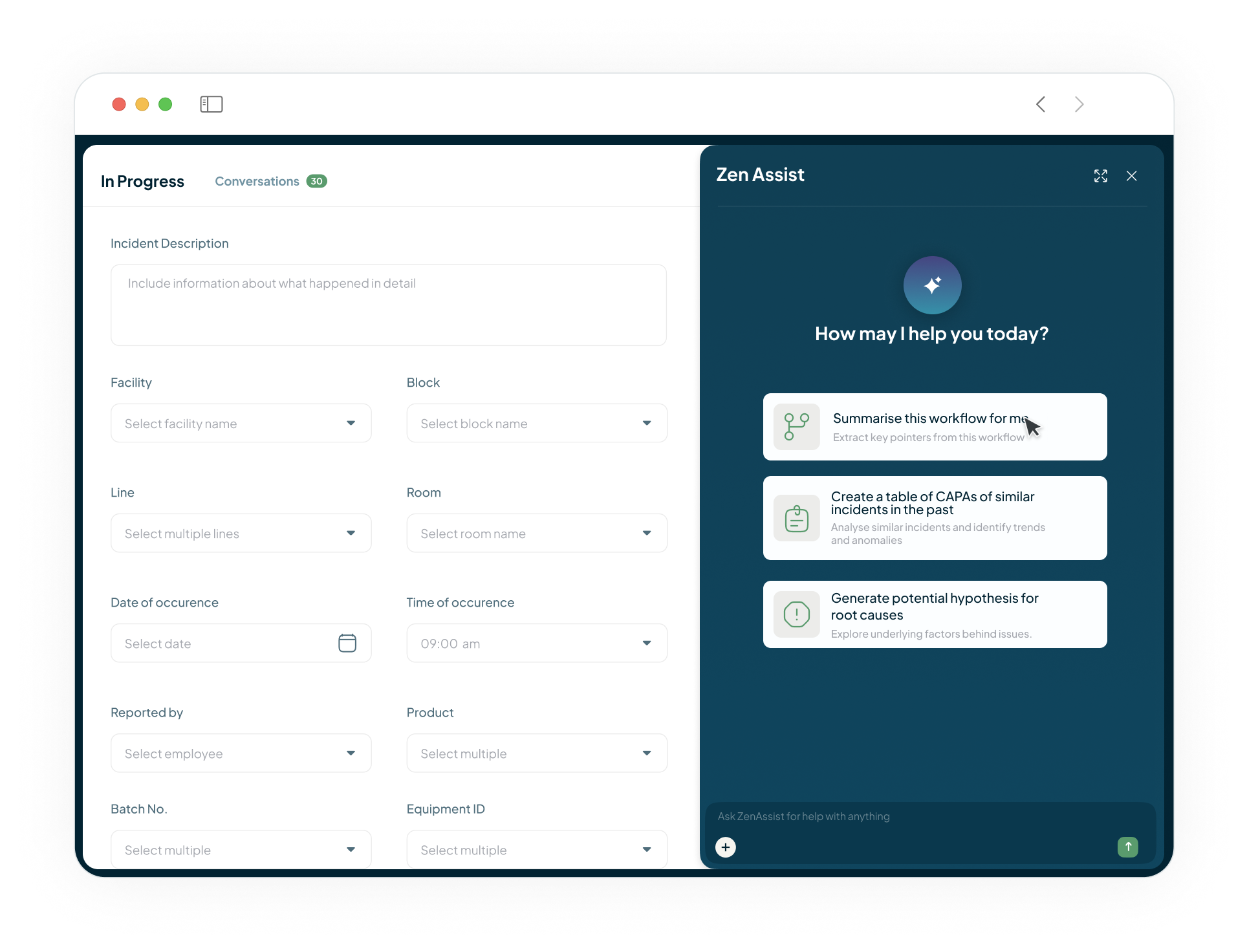
Streamline Your Entire Quality & CAPA Lifecycle
Our AI-native QMS provides a single, unified hub to manage all quality events—from deviations and non-conformances to investigations, change controls, and CAPAs. Flexible workflows adapt to your process, and AI assistance helps close out investigations and perform impact analysis for change management faster.

Guarantee 21 CFR Part 11 Compliance & Data Integrity
Our GxP-compliant platform offers immutable audit trails, secure e-signatures, and fine-grained access controls. This ensures tamper-proof records and full compliance with 21 CFR Part 11 and ALCOA+ data integrity, making you "always audit-ready."

